Try these at home with an adult- they are like the examples on the wonder table
Mixing Oil and Water
Some things just don't get along well with each other. Take oil and water as an example, you can mix them together and shake as hard as you like but they'll never become friends.....or will they? Take this fun experiment a step further and find out how bringing oil and water together can help you do your dishes.
|
What you'll need:
|
Instructions:
- Add a few drops of food colouring to the water.
- Pour about 2 tablespoons of the coloured water along with the 2 tablespoons of cooking oil into the small soft drink bottle.
- Screw the lid on tight and shake the bottle as hard as you can.
- Put the bottle back down and have a look, it may have seemed as though the liquids were mixing together but the oil will float back to the top.
Parents need to explain!
What's happening?
While water often mixes with other liquids to form solutions, oil and water does not. Water molecules are strongly attracted to each other, this is the same for oil, because they are more attracted to their own molecules they just don't mix together. They separate and the oil floats above the water because it has a lower density.
If you really think oil and water belong together then try adding some dish washing liquid or detergent. Detergent is attracted to both water and oil helping them all join together and form something called an emulsion. This is extra handy when washing those greasy dishes, the detergent takes the oil and grime off the plates and into the water, yay!

|
 Procedure Procedure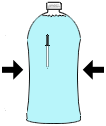 Take the empty soda bottle and fill it completely with water. Fill the water glass with water and place the medicine dropper in the glass. Get some water inside the dropper by squeezing the rubber bulb while the end is in the water. You want to get the dropper to just barely float upright in the water. Once you've done this, place the dropper in the soda bottle and screw on the cap tightly. Don't allow much air to be between the top of the bottle and the cap. Gently squeeze the bottle. As you squeeze, the diver will dive (sink) to the bottom of the bottle. If you stop squeezing, the diver floats back to the top. Take the empty soda bottle and fill it completely with water. Fill the water glass with water and place the medicine dropper in the glass. Get some water inside the dropper by squeezing the rubber bulb while the end is in the water. You want to get the dropper to just barely float upright in the water. Once you've done this, place the dropper in the soda bottle and screw on the cap tightly. Don't allow much air to be between the top of the bottle and the cap. Gently squeeze the bottle. As you squeeze, the diver will dive (sink) to the bottom of the bottle. If you stop squeezing, the diver floats back to the top. | |
 What's going on? What's going on?This experiment demonstrates the property of buoyancy. An object is buoyant in water due to the amount of water it displaces or 'pushes aside'. If the weight of water that is displaced by an object in water exceeds the weight of the object then the object will float. As you apply pressure to the bottle, you apply pressure to the air bubble in the dropper reducing its size. As the bubble's size reduces, the dropper becomes less buoyant and begins to sink. Release the pressure on the bottle and the dropper begins to rise back to the top. Fish keep themselves from either sinking or floating to the surface by using muscles to squeeze or relax a small sac (with a small air bubble inside) in their bodies. By squeezing the sac smaller, the fish will sink. By relaxing their muscles, the sac increases in size, displaces more water, and a fish will begin to rise to the surface. Man uses this same principle to control the buoyancy of a submarine. By pumping water in and out of tanks stored in the submarine, a submarine can be made to rise and sink. | |
 Mess Factor Mess FactorJust the possibility of getting a little wet. | |
 Things to Remember Things to RememberCartesian is a term that was named after René Descartes, a French scientist, mathematician, and philosopher. He laid the foundations of analytical geometry, algebra, and other subjects such as buoyancy and pressure. René Descartes sought truth by first doubting everything, even his own existence. But he concluded that in order to be able to doubt his existence, he must exist. Descartes accepted traditional Christian beliefs, and he deduced the existence of God and then the existence of the physical world. |
Oil and water

What's happening: First, the science behind a hard-boiled egg: Egg whites are made of water and proteins. Proteins are made of long chains of amino acids, but in an egg the chains are clumped tightly together in individual spheres. (These are called "globular proteins.") When the egg is heated, the proteins and water molecules begin to move faster. As they move and collide with each other, the individual protein chains start to "unravel," eventually bonding loosely with other protein chains, forming a network of protein with water trapped inside. The consistency has changed from runny egg white to a soft solid! So how does this squishy-but-solid egg get mysteriously pushed inside the bottle? The answer is all about air pressure. When you first set the egg on the bottle, the air pressure inside the bottle matched the air pressure outside, so nothing happened. When you dropped the burning paper into the bottle, it caused the air inside to heat up and expand rapidly. That expanding air pushed the egg aside and escaped from the bottle; that's why you saw the egg vibrating. When the fire consumed all the oxygen inside the bottle, the flame went out and the remaining air in the bottle cooled down. Cool air takes up less space, exerting less pressure inside the bottle. (The egg acted as a seal to prevent outside air from getting in to fill the extra space.) The result was an unbalanced force—the force of the air pushing on the egg from outside the bottle was greater than the force of the air pushing up on it from inside the bottle. Voila - the egg was pushed into the bottle!
No comments:
Post a Comment
We would love to read your comments ....Room 15 invites you to enjoy our blog.